Elucidation of the molecular mechanisms by which general anesthetics act is an important goal in clinical medicine and neuropharmacology. It is necessary for the development of safer and more specific anesthetic agents, and for the safe use of available agents while minimizing toxicity.
Electrophysiological evidence indicates that general anesthetics act primarily on synaptic transmission without major effects on axonal conduction or neuronal excitability. At clinically relevant concentrations, most general anesthetics depress excitatory synaptic transmission while some, including propofol and barbiturates, facilitate inhibitory synaptic transmission.
There is good evidence for both presynaptic and postsynaptic mechanisms for these effects, including:
- Presynaptic reductions in excitatory neurotransmitter release
- Inhibition of postsynaptic responses to excitatory neurotransmitters
- Facilitation of postsynaptic and extrasynaptic responses to inhibitory neurotransmitters
However, the precise molecular mechanisms involved in these effects are unknown.

Rat hippocampal neurons (14 DIV) transfected with vGlut-pHluorin during electrical stimulation [50 action potentials at 50 Hz (open circle).
Sodium channels as anesthetic targets
Volatile anesthetics (VA) decrease presynaptic Ca2+ and processes that determine nerve terminal excitability/membrane depolarization or regulate presynaptic Ca2+ are targets for VA effects on synaptic vesicle exocytosis. Identification of these targets and their roles in determining presynaptic sensitivity to anesthetics is critical to understanding synaptic anesthetic pharmacology sufficiently to optimize the pharmacological specificity of CNS depressant drugs.
Voltage gated Na+-channels (Nav) control neuronal excitability, action potential driven Ca2+ influx, and Ca2+-dependent neurotransmitter release. Nav subtypes are differentially inhibited by VAs and we examine how heterogeneity of presynaptic Nav subtype expression underlie transmitter-specific differences in sensitivity to VA.
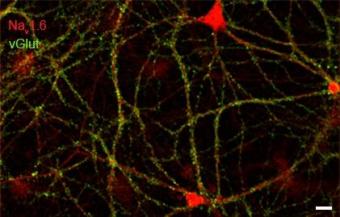 Rat hippocampal neurons (21DIV) dual immuno-labeled with Nav1.6 and vGlut. Scale bar = 200µm
Rat hippocampal neurons (21DIV) dual immuno-labeled with Nav1.6 and vGlut. Scale bar = 200µm
Rat hippocampal neurons (21DIV) dual immuno-labeled with Nav1.6 and vGlut. Scale bar = 200µm
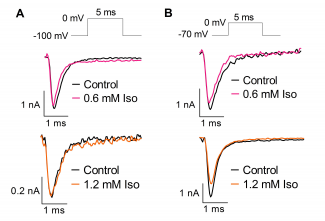
Effect of isoflurane (Iso) on Na+ currents from HEK293T cells transfected with human Nav1.1 and held at -100mV (A) or -70mV (B).
Interneuron diversity
An inherent problem in comparing differences in presynaptic pharmacology between glutamatergic and GABAergic neurons is the diversity in GABAergic interneuron subtypes which vary in their key neurotransmission-related proteins. Interneurons modulate nearly every aspect of neuronal excitability and network function with cell-specific roles. Thus, understanding cell-specific anesthetic actions on interneuron output is critical to understanding the neurophysiology of the anesthetized state and we utilize multiple methods for interneuron identification and characterization.
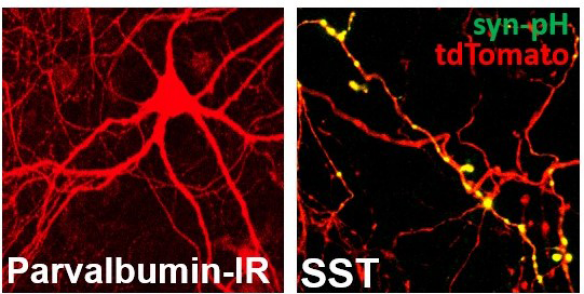
PV+ neuron identified by immunoreactivity (left). SST+ axon, transfected with syn-pH (yellow) in SST::Ai14 cultures (right).
Volatile anesthetic effects on intracellular Ca2+ dynamics influence synaptic vesicle exocytosis
Ca2+ influx through presynaptic voltage-gated Ca2+ channels (Cav) is required for neurotransmitter release. VA effects on synaptic transmission and neurotransmitter release are mediated by inhibition of presynaptic Ca2+ influx, but the mechanisms by which VAs reduce presynaptic Ca2+ is not fully understood. Ligand-gated ion channels, including Nav and Cav, and intracellular Ca2+ stores, are leading candidates for VA effects on intracellular Ca2+.
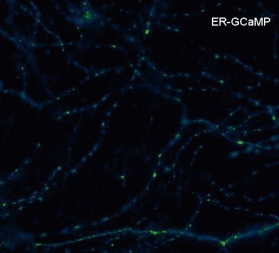
Rat hippocampal neurons (14 DIV) transfected with ER-GCaMP during electrical stimulation [20 action potentials at 20 Hz (open circle)}.
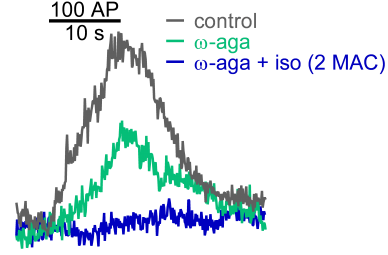
Fluorescence traces of a single bouton from a neuron transfected with vGlut-pHluorin. w-Aga blocked P/Q-type mediated exoctytosis; 2 MAC isoflurane inhibited residual N-type mediated exocytosis.
Volatile anesthetics directly bind Nav
Advances in structural biology have begun to reveal drug binding sites on Nav, but sites of VA interactions with mammalian Nav are unavailable largely due to a lack of detailed structural information. Although an eukaryotic Nav has not been crystallized, prokaryotic Nav provides an alternate approach to obtain rigorous structural and functional information on VA interactions with Nav. We have observed that isoflurane inhibits the prokaryotic channel NaChBac expressed in HEK cells. While NaChBac has not been crystallized, the homologous channel NavMs has, so we will pursue co-crystallization of NavMs with VAs to identify VA binding sites with co-investigator Prof. Bonnie Wallace (Birbeck College).
We will further use electrophysiology to determine the functional impact and validate VA binding sites in NavMs identified.
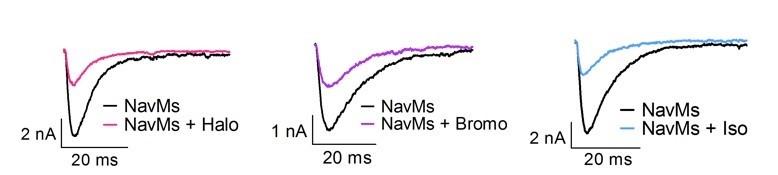
Effects of halothane (0.4mM), bromoform (0.2mM), or isoflurane (0.6mM) (~2 MAC each) on Na+ currents of NavMs expressed in HEK293T cells held at -200mV with 50ms test pulses to -50mV.

