Upstream of spine dynamics, anesthetics potently modulate synaptic activity to produce cell-type specific effects on synaptic structure and function; depression of excitatory signaling, loss of spine density and size, and cognitive impairments are found to be associated with attenuation of activity dependent brain-derived neurotrophic factor (BDNF) release by anesthetics. Moreover, humans with a common single nucleotide polymorphism in the BDNF gene (Val66Met) show altered synaptic plasticity, which may increase susceptibility of cognitive decline following exposure to anesthesia.
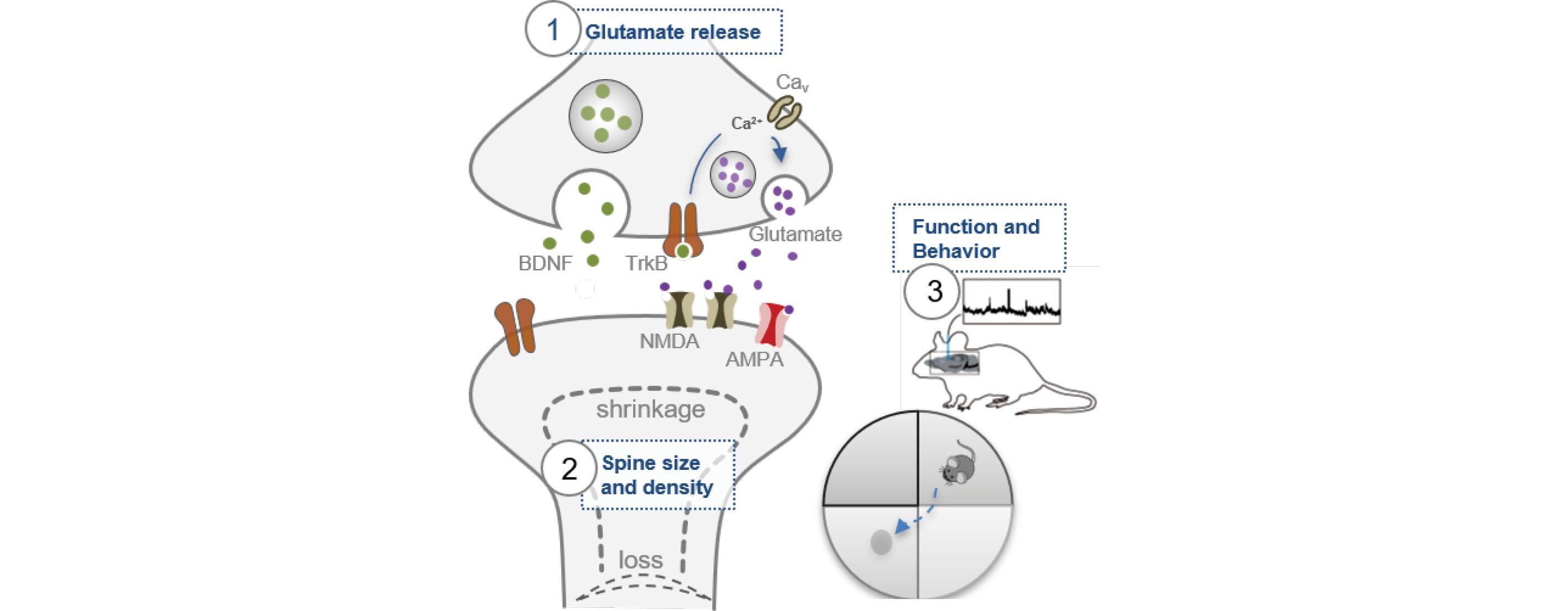
Diagram showing contribution of BDNF signaling to activity-dependent neurotransmission (1), dendritic spine area and density (2), and learning and memory (3). We aim to identify the role of a BDNF SNP in anesthetic-induced alterations in excitatory transmission, spine structure, and neuronal activity and function.
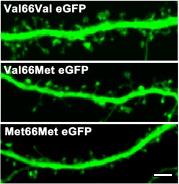
Dendritic arbor of Val66Val (wild-type top), Val66Met (heterozygous, middle) and Met66Met (homozygous, bottom) mice neurons (21DIV) transfected with eGFP. Scale bar = 5μm.
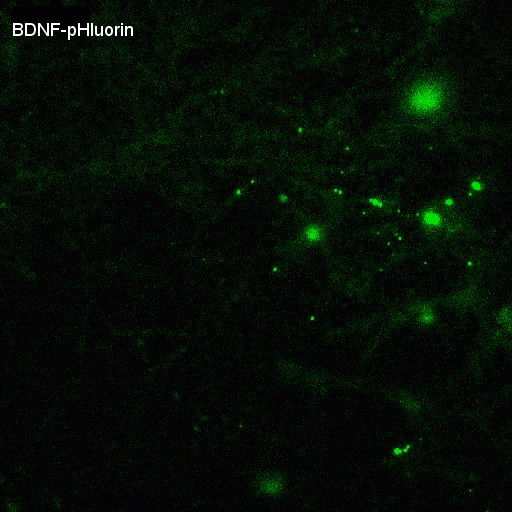
Video of rat hippocampal neurons (14DIV) showing asynchronous exocytosis of BDNF-pHluorin during theta-burst stimulation. Duration of stimulation indicated by closed circle.