Advances in structural biology have begun to reveal drug binding sites on Naᵥ, but sites of anesthetic interactions with mammalian Naᵥ are unavailable due to a lack of detailed structural information. Although eukaryotic Naᵥ has not been crystallized, prokaryotic Naᵥ provides an alternate approach to obtain rigorous structural and functional information on anesthetic binding to Naᵥ. We have shown that isoflurane inhibits the prokaryotic channel NaChBac and the homologous channel NavMs. We are currently investigating this interaction by co-crystallization of NavMs with volatile anesthetics to identify binding sites in collaboration with Prof. Bonnie Wallace (Birkbeck College). We will use electrophysiology to determine the functional impact and validate anesthetic binding sites identified in NavMs.
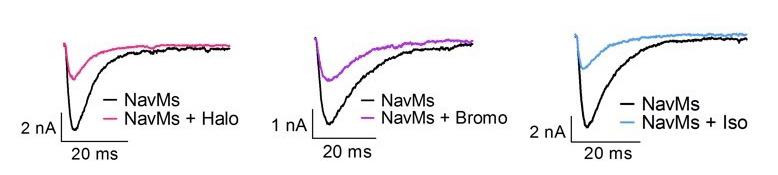
Effects of halothane (0.4 mM), bromoform (0.2 mM), or isoflurane (0.6 mM) (~2 MAC each) on Na⁺ currents of NavMs expressed in HEK293T cells held at -200 mV with 50 ms test pulses to -50 mV.