Volatile anesthetics decrease presynaptic Ca²⁺ entry, and processes that determine nerve terminal excitability and membrane depolarization that regulate presynaptic Ca²⁺ are targets for anesthetic effects on synaptic vesicle exocytosis. Identification of these targets and their roles in determining presynaptic sensitivity to anesthetics is critical to understanding synaptic anesthetic pharmacology sufficiently to optimize their pharmacological specificity.
Voltage gated Na+-channels (Naᵥ) control neuronal excitability, action potential driven Ca²⁺ influx, and Ca²⁺-dependent neurotransmitter release. Naᵥ subtypes are differentially inhibited by volatile anesthetics, and we have shown that heterogeneity in presynaptic Naᵥ subtype expression underlies transmitter-specific differences in sensitivity to anesthetics.
Rat hippocampal neurons (14 DIV) transfected with vGlut-pHluorin during electrical stimulation [50 action potentials at 50 Hz (open circle)].
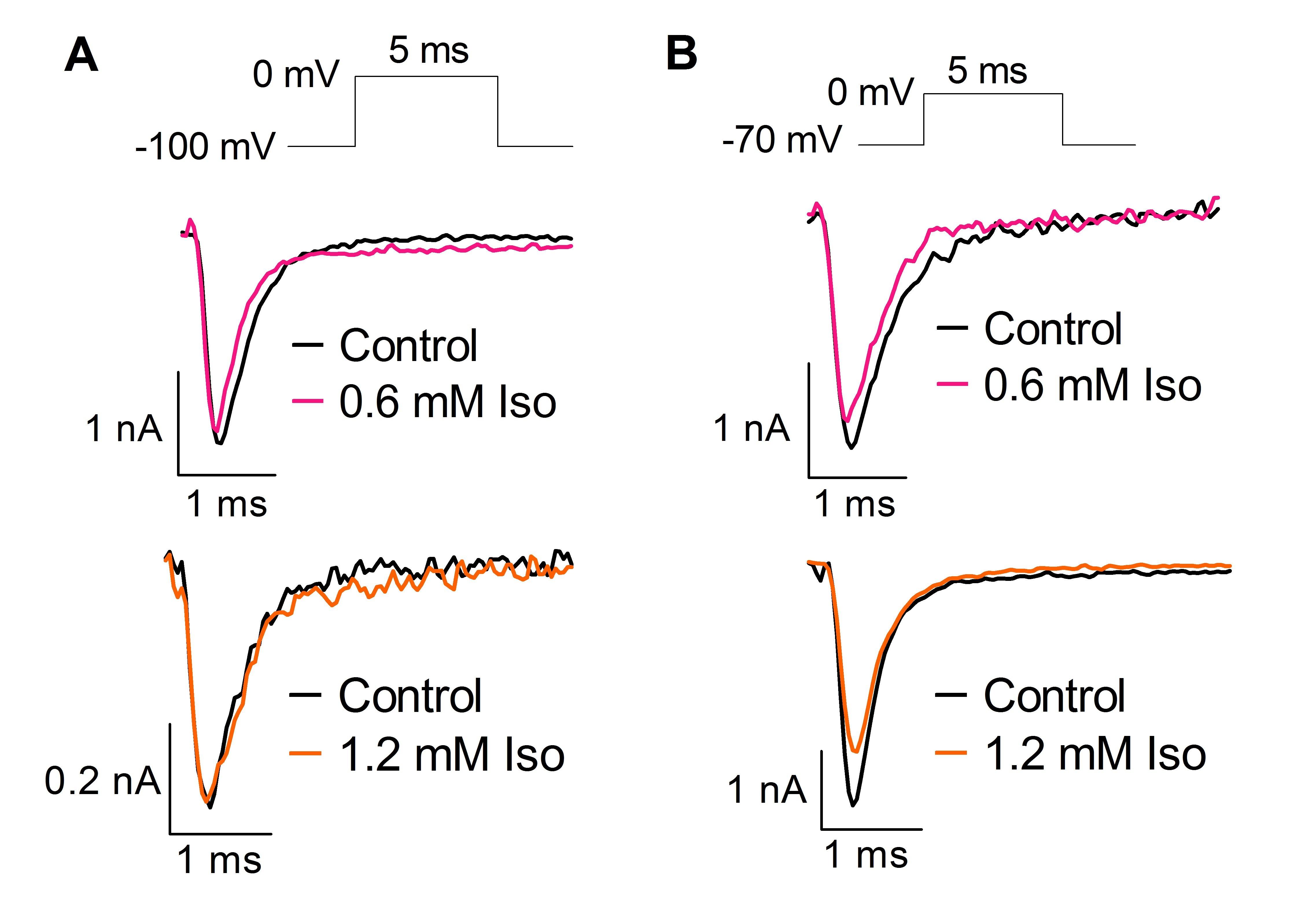
Effect of isoflurane (Iso) on Na⁺ currents from HEK293T cells transfected with human Naᵥ1.1 and held at -100 mV (A) or -70 mV (B).
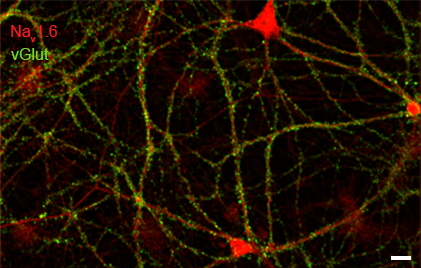
Rat hippocampal neurons (21DIV) immunolabeled for Naᵥ1.6 and vGlut. Scale bar = 200µm.